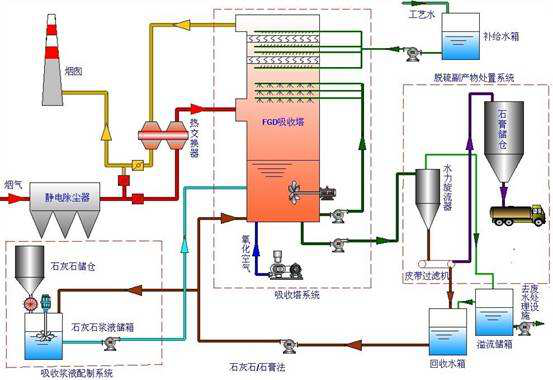
Introduction of limestone gypsum desulphurization process:
1. Absorption process
The boiler flue gas enters the absorption tower after the dust removal. The circulating slurry flowing upward and downwards in the absorber tower is washed in countercurrent mode. The circulating slurry is ejected into the absorption tower through the nozzle set in the spray layer to remove SO2, SO3, HCL and HF at the same time. At the same time, the air produced by the by-product of the "forced oxidation process" is oxidized into gypsum and consumes the limestone as an absorbent. The circulating slurry is transported upward through the slurry circulation pump to the spray layer, and atomized by the nozzle can make the gas and liquid fully contacted. Each pump is usually connected to its own spray layer, that is, the unit system is usually adopted.

In the absorber, limestone and sulfur dioxide react to form gypsum. This gypsum slurry is discharged through gypsum slurry pump and enters gypsum dewatering system. The dehydration system mainly includes gypsum hydrocyclone (as a primary dehydration equipment), slurry distributor and vacuum belt dehydrator.

The purified flue gas flows through the two stage demister to remove mist, and the droplet droplets carried in the clean flue gas are removed here. At the same time, wash the mist eliminator with process water from time to time according to specific procedures. There are two purposes for cleaning the mist eliminator, one is to prevent the mist eliminator from blocking up, and the other is to flush the water at the same time as a supplementary water to stabilize the absorber's liquid level. Two.

Two. Chemical equation of desulphurization reaction
1.SO2+H2O - H2SO3----------------------- absorption
2.CaCO3+H2SO3 to CaSO3+CO2+H2O------- neutralization
3.CaSO3+1/2O2 to CaSO4------------------ oxidation
4.CaSO3+1/2H2O to CaSO3? 1/2H2O---------- crystal
5.CaSO4+2H2O to CaSO4? 2H2O--------------- crystal
6.CaSO3+H2SO3 - Ca (HSO3) 2---------------PH control
At the same time, HCL and HF react with CaCO3 to form CaCl2 or CaF2 in flue gas. PH in the absorption tower by injection
Limestone slurry is regulated and controlled, with a general pH value of 5.5-6.2.