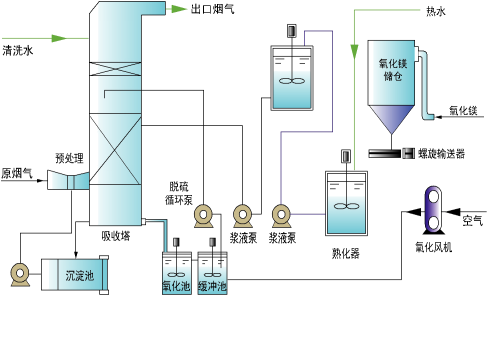
First, process introduction
Magnesium desulphurization is made from Magnesium Oxide (MgO) as a raw material. The slurry is formed by curing the slurry as a desulfurizer, and it is exposed to the flue gas in the absorption tower. The sulfur dioxide in the flue gas is reacted with the Magnesium Oxide in the slurry to be removed. The final reaction product is the sub Magnesium Sulfate, and the oxidation aeration produces the Magnesium Sulfate solution through the oxidation aeration. Magnesium Sulfate can be used to produce struvite or evaporation, concentration and crystallization to produce Magnesium Sulfate crystals. It can be used as foliar fertilizer.

Two. The main chemical reactions in the process of desulphurization
MgO+H2O=Mg (OH) 2
Mg (OH) 2+SO2=MgSO3+H2O
Mg (HSO3) 2, mg / O 3 + H 2 O + Su 2 = mg
MgSO3+1/2O2=MgSO4

Three, the characteristics of Magnesium Oxide desulphurization:
Magnesium Oxide desulfurization is a promising desulfurization process. The technology is relatively mature, and the raw materials are abundant. The reserves of Magnesium Oxide in China are very considerable. Therefore, Magnesium Oxide can be used as desulfurizer in desulfurization systems of various units.

1, the magnesium method has the advantages of less investment, low operating cost, high desulfurization efficiency, simple structure, and can reduce two times of pollution.
2. The greatest advantage of magnesium desulphurization relative to the calcium method is that the problem of scaling and clogging of the equipment is not systematic. It can ensure the safe and effective operation of the whole desulfurization system. At the same time, the pH value of the magnesium method is controlled between 6 and 6.5, and the corrosion problem of equipment is also solved to a certain extent under this condition.